Engineered Viruses Make Neurons Glow and Treat Brain Disease
Neuroscientists can now make precise genetic tweaks to the neurons that are most affected by brain diseases such as Parkinson’s, Huntington’s and ALS
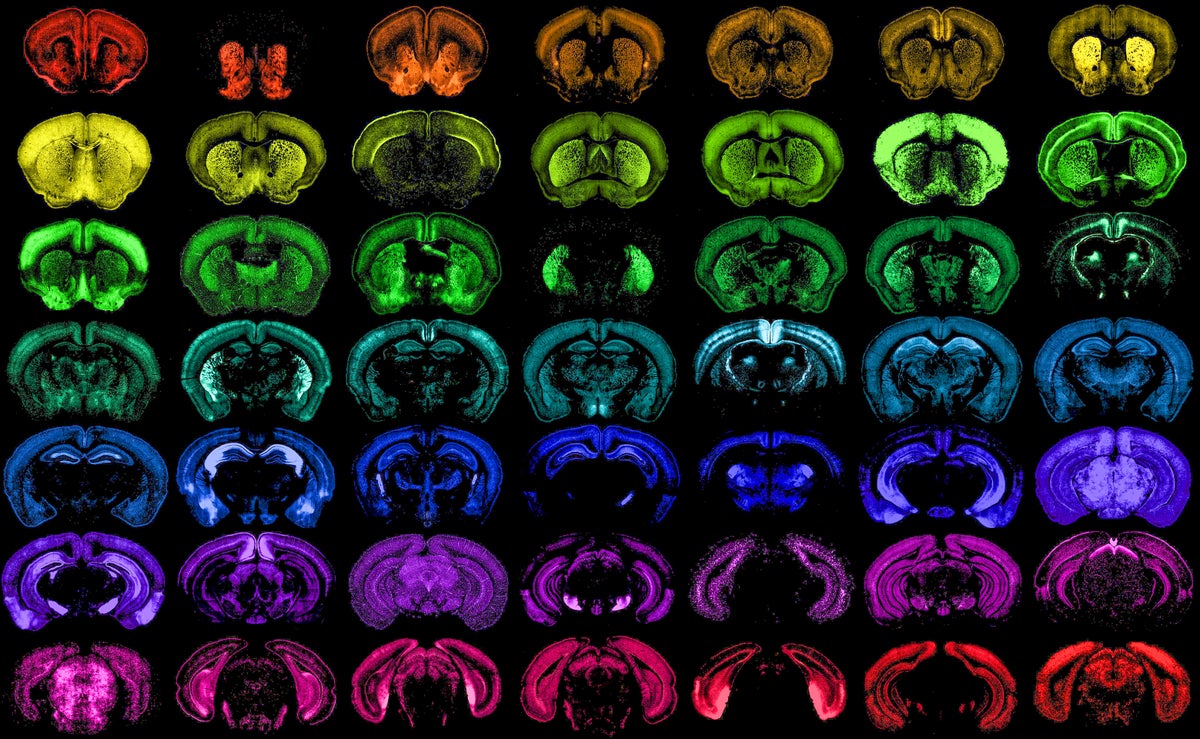
Different populations of cells in the mouse brain, each one targeted with high specificity by one of the many new genetic tools developed at the Allen Institute.
The brain is like an ecosystem—thousands of different types of cells connect to form one big, interdependent web. And just as biologists document species of plants and animals, neuroscientists have spent decades identifying different “species” of neurons and other brain cells that support them. They’ve found more than 3,000 cell types spread throughout the brain, including chandelier neurons surrounded by branching arms, pyramidal neurons with far-reaching nerve fibers and star-shaped astrocytes that help neurons form new connections with one another.
This newfound diversity is not only a beautiful picture for neuroscientists—it’s also key to understanding how the brain works and what goes wrong in certain brain diseases. From Parkinson’s disease to schizophrenia, many brain disorders stem from specific types of brain cells.
“As long as I’ve been doing neuroscience, it’s been a goal of researchers to have brain-cell-type-targeting tools,” says Jonathan Ting of the Allen Institute, a nonprofit research center in Seattle. Now they have them in spades. In a fleet of eight studies funded by the National Institutes of Health and published last week, scientists from 29 research institutions found and tested more than 1,000 new ways to home in on specific cell types, no matter where they are in the brain.
If you’re enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
The technique behind these tools uses non-disease-causing viruses (called adeno-associated viruses, or AAVs) to deliver genes directly to specific neurons. This can make the cells do almost anything. Scientists can turn them off, activate them, “light them up like a Christmas tree” with glowing proteins or deliver gene therapies right to them, says Ting, senior author of one of the new studies. The researchers have tested the technique only in nonhuman animals, but the bulk of the tools work across mammal species and would likely work in humans, too. Similar, less-targeted AAV gene therapies are already approved for treating spinal muscular atrophy and are being tested in clinical trials for Huntington’s disease.
Every type of brain cell is like a unique creature. Scientists have categorized the cells based on their shape, location and electrical properties—and, more generally, based on the genes they express most out of an organism’s full library of DNA. By expressing certain genes, these cells carry out specific actions, such as building specialized proteins. If researchers can identify a unique snippet of genetic code that is activated just in those cells, they can use that snippet to target them.
Next, they attach this genetic snippet, called an enhancer, to an AAV that has been gutted of its viral DNA. They can fill the viral husk with specific genes to deliver to those cells. The now-filled husks enter the bloodstream like a fleet of delivery shuttles, bypassing the blood-brain barrier, but are only able to activate their genetic cargo in cells with the enhancer.
Previously, these enhancer AAVs had been developed in a slow trickle by different labs, but “now we have thousands of tools” to tweak specific cell types, says Bosiljka Tasic, director of molecular genetics at the Allen Institute and senior author of one of the new studies.
Researchers can load these AAV shuttles with all sorts of different genes to answer different questions. In some cases, even just seeing the neurons in action is cause for celebration: “Some of them are very rare cells that you wouldn’t find randomly by poking around in brain tissue,” Ting says. To observe them, researchers can introduce a gene that makes a glowing protein that lights up elusive neurons from the inside to reveal their structure and how they connect with other brain cells.
Researchers can also control how certain brain cells fire and turn their activity up or down to see how the change impacts an animal’s behavior. To do this, researchers insert a gene into the target cells that creates a light-sensitive protein called an opsin; then they can shine specific wavelengths of light on the brain to make those cells fire on command. Ting’s team used this technique, called optogenetics, to stimulate certain cells in the striatum of mice. When the researchers stimulated those cells on just one side of the brain, the mice began moving more on one side of their body than the other, causing them to go in circles.
These interventions are reversible and repeatable. “That’s the part that’s really satisfying for neuroscientists,” Ting says. “You can turn them off, turn them back on and then see how that affects the brain circuit.”
It’s “so much better and also so much more informative” than destroying whole parts of a mouse brain to see what happens, as is the case with much neuroscience research from the past century, Tasic says. “That brain region may have a hundred different cell types,” so being able to activate and inactivate them more precisely will reveal more information about how these circuits work, she says.
So far, the new enhancer AAVs have been tested in mice, rats and macaques. “We keep trying more and more species,” Ting says. “We haven’t even figured out what’s the limit.”
And that brings us to humans. “That’s really the answer to the question ‘Why do we care?’” he says. “We have built strong evidence that some of these tools—maybe not all of them, but many of them—may work across species into humans and could represent the start of a new therapeutic vector development that could be used to more finely treat debilitating brain disorders.”
For these treatments, enhancer AAVs could deliver gene therapy right to the brain cells that need it. The best candidates for this technique are neurodegenerative diseases, such as ALS, Parkinson’s disease and Huntington’s disease. Researchers are currently working on AAV gene therapies for these conditions and others that target whole regions of the brain rather than specific types of brain cells. Trials of these therapies indicate that they are largely safe. “We now have lots of good examples of AAV being used,” McFarland says. “We have [a] good safety record for that.”
“There’s a lot that we still don’t understand about neurodegenerative diseases,” he adds, and these little viral shuttles will allow scientists to make those discoveries that enable new treatments. While each of these brain disorders is unique, cracking one of them might help scientists crack the others, too, McFarland says: “I wholeheartedly believe that.”
Allison Parshall is an associate editor at Scientific American covering mind and brain. She writes the magazine’s Contributors column and weekly online Science Quizzes. As a multimedia journalist, she contributes to Scientific American‘s podcast Science Quickly. Parshall’s work has also appeared in Quanta Magazine and Inverse. She graduated from New York University’s Arthur L. Carter Journalism Institute with a master’s degree in science, health and environmental reporting. She has a bachelor’s degree in psychology from Georgetown University. Follow Parshall on X (formerly Twitter) @parshallison
Source: www.scientificamerican.com