Can Your Microbiome Affect Your Mood?
Scientists are uncovering how your gut might be shaping your thoughts, feelings and cravings.
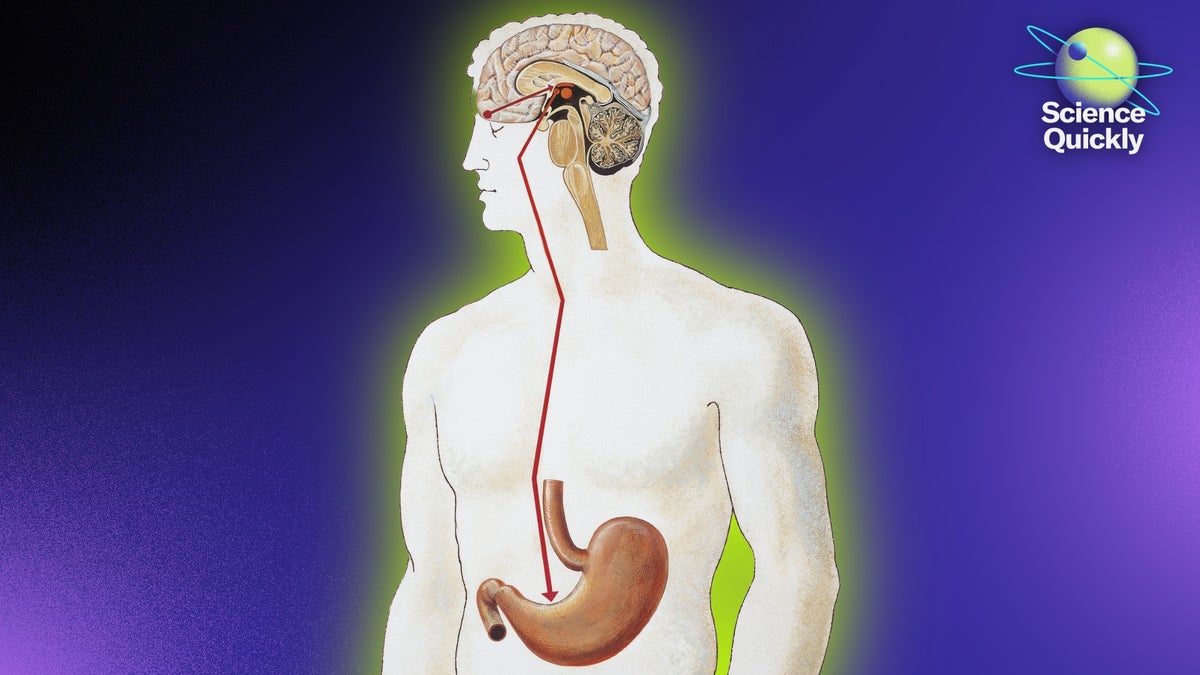
An illustration of a man revealing his brain and stomach with an arrow drawn between them on a purple background
This episode was made possible by the support of Yakult and produced independently by Scientific American’s board of editors.
Rachel Feltman: For Scientific American’s Science Quickly, I’m Rachel Feltman.
People often talk about having “gut feelings,” but new research suggests there may be more to the idiom than we thought. Scientists are finding that specialized cells in our intestines can send signals directly to the brain, potentially influencing appetite and even mood.
If you’re enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
Recent studies hint that our microbiomes could play a role in this communication system, though researchers are still trying to understand exactly how these interactions work and what they mean for our health.
Thanks so much for coming on to chat with us today.
Maya Kaelberer: It’s my pleasure. I’m happy to be here.
Feltman: So you recently co-authored a study that looks at the gut-brain connection a little bit. Could you tell us a little bit about why scientists are interested in that and what we know about it so far?
Feltman: Mm.
Kaelberer: And so understanding the molecular and cellular and neuronal connections between the gut and the brain is gonna help us better understand, like, this relationship that we have, that we have these gut feelings, right?
Feltman: Yeah.
Kaelberer: We know they’re there [laughs].
Feltman: Well, and beyond, you know, hanger, which is obviously a great example, what are some conditions that have been connected to the gut that might surprise people?
Kaelberer: So when I was in my postdoc we discovered that there was this direct connection between these cells in the surface of the gut, we call them neuropod cells, and neurons that communicate directly—they reach directly into the brain. And so we call this as—our “gut sense,” and the number-one question I would always get was: Who cares? Like, what [laughs], you know, what is our gut possibly telling us that our mouth and our nose did not already tell us about the food that we ate?
And so we delved into this a little bit more in some previous publications with regards to sugar sensing, and I use this example ’cause it’s really salient in my own life, which is that I like artificial sweetener in my coffee.
Feltman: Mm.
Kaelberer: And I don’t like regular sugar because regular sugar just feels heavy to me, and I want that, like, artificial sweetener. It kind of keeps me going. I can be caffeinated. I can be, like, on the go. I’m not gonna, like, sit down and take a nap afterwards. And so we know that these two stimuli feel different in our gut. And what we found is that these neuropod cells are actually distinguishing between the two stimuli, between real sugar and artificial sweetener. And they release different signals, and then the signal for sugar actually drives the animal to consume the sugar over the artificial sweetener.
So now we take it back to my coffee preference, and suddenly, I’m like, “Well, this makes sense. I like the artificial sweetener because I don’t want that heaviness.” And that heaviness is telling me that that food was gratifying or that food was satisfying; there was some kind of value associated with that that’s gonna help me survive in nature. And so then this is now this communication system of, like, “Oh, our gut sense is telling us something about the food we eat past whether or not it tastes good. It’s telling us a little bit about the value of what we’re consuming.”
Feltman: So let’s get into the latest study. What were you guys looking for, and what did you find?
Kaelberer: Yeah, so the microbiome is everywhere. We’re always hearing about how the microbiome affects a lot of our behaviors, and there’s all this really cool work that’s coming out showing that there are these different species, they can affect your mood, but we don’t really know what that connection is. And so when we found that these neuropod cells are responding to nutrients, we thought, “Oh, well, in the gut there’s this microbiome population. I wonder if the microbiome is actually signaling via these neuropod cells.”
And so it turns out what we found is they do, hence the study. And they’re doing it in this really interesting way. So what they’re doing is they’re sensing this protein that’s on the tail of the bacteria, and this is any bacteria that has a tail—it has to swim around, has a tail—and this protein, so it’s pretty widely expressed across all these different microbes. And what [a neuropod cell is] sensing is: it’s sensing that protein, and that protein tells the animal when it’s being sensed that the animal should eat a little bit less and when the protein isn’t there that the animal should eat a little bit more.
And you can think, like, this might be counterintuitive because aren’t we against bacteria [laughs], right? Like, it, it infects us all the time. We don’t want it to grow. But that’s not always true. We want our microbiome to grow. We want it to be healthy. We want it to maintain a good population size because it has all these benefits. And so what we found is that this is actually a circuit, via these neuropod cells, and it’s a direct connection that allows the microbiome and the host to somehow communicate about what its food needs are.
Feltman: Very cool. So what do you think the implications of those findings are?
Kaelberer: So I think that the implications of these findings open a lot of possibilities, right? If we know—it’s, like, figuring out: What’s the language they’re using to communicate? And now, once we know what the language they’re using is, now you can imagine: “Okay, are there nutrients that affect the signaling pathway? Are there probiotics that influence it more or less? Are there other signals, for instance, from the microbes, not just this tail protein but other things that are actually influencing different types of behavior?”
Like, there are some studies that are looking at social interaction and showing that there’s a certain species of microbes that’s associated with being more sociable, but we still don’t know what that direct pathway is. And so this is just kind of the beginning of, like, well, what is this, this “neurobiotic sense” is what we’re calling it, because it’s this whole new sense that’s all about: How do we communicate with our microbiome, and how does our microbiome communicate with us?
Feltman: So what are your next steps research-wise?
Kaelberer: So research-wise the next steps are—I actually already mentioned them a little bit. We’re interested to know how it is that the food that we’re eating is being processed by those microbes in order to adjust that signal. So you could imagine, like, a high-fat or a high-sugar diet—I have this pet theory: I think that people with a sweet tooth actually have microbes with a sweet tooth and that there’s some kind of communication going on there that’s saying, like, “Hey, maybe eat a little more sugar. We want a little more sugar down here.” Or, like, a high-fat diet, this is another, like, thing that we study a lot is: How is it that our diet, or highly processed food, how is this actually influencing that ecosystem that we contain in our gut?
Feltman: How far out do you think we are from being able to take advantage of some of these gut-brain connections by manipulating our microbiome?
Kaelberer: Rachel, that is a great question because we talk a lot about the science, we talk about the initial discoveries. I’m gonna give a small example, which is Ozempic. You’ve probably heard of Ozempic.
Feltman: Mm-hmm.
Kaelberer: It’s a GLP-1 agonist. GLP-1 is what’s called a satiety hormone. This satiety hormone was discovered in animals. And so you think about it: Okay, we discovered it in animals. We tested it, we tested its function and basic research. We moved on into human research. And then the first Ozempic drugs, if I’m getting my dates right, were in the teens, was when they came out. And so now we’re talking about, like, 30 years of research that had to go into this really key discovery that was made before we had the implications in human health.
Feltman: Right.
Kaelberer: I don’t think it’s gonna take that long because there’s a lot of other stuff that we’ve built on.
Feltman: Mm-hmm.
Kaelberer: But I think it’s important to know, like, all right, you’ve gotta test the mechanism. We don’t just give people anything [laughs], right? We have to go through the process of, like, testing it out, testing what we know about it and then testing, like, are there other drugs, say—so this is another common way to do it—are there other drugs that are already approved in humans that are influencing this new pathway that we discovered and are having this positive effect? And so that pathway is actually a quicker way to get to human implications.
And so one thing that I thought was really cool about the study that we did was that it’s an experiment that’s, at, at kind of the end, was testing this in what are called germ-free mice. So germ-free mice are mice that have never seen a microbe in their lives. They grow up without bacteria at all. They’re completely free of it. And these mice are a little weird; turns out we need them. But what we did is we took these germ-free mice and we tested whether that tail protein, this flagellin signal, had the same effect in these mice. And it did; it actually—it decreased their feeding.
And why do I point that out? It seems like, “So what? Who cares?” Right? But this finding, I think, is actually really interesting because what it implies is that the mouse did not need prior experience in order for this circuit to actually exist. And when that happens, usually what that means is this is because the circuit evolved; we evolved with our microbiomes in order to communicate. This isn’t just some kind of reaction that we’re learning based on what microbes come into our body.
And so I think understanding that kind of aspect of it puts a different spin on the research ’cause now we’re talking about, okay, this is a lifelong interaction that we’ve had, so now we know we can’t just get rid of it, right? You can’t just take the microbes out. We actually have to work with the microbes in order to improve health.
Feltman: Yeah.
Kaelberer: As you can probably tell I’m very passionate about the work that I’m doing, and I just think that we’re at the forefront of so many really cool discoveries. So I, I always ask, Rachel, and I’m gonna ask you this question: Now that you know what a gut sense is, think about your last meal. How did you feel? How did your gut feel about what you last ate?
Feltman: I think my gut felt pretty good about what I last ate. It was a salad. There was a lot, a lot of variety in there. There was a lot going on. So I don’t think my gut felt bad about it [laughs].
Kaelberer: But now you’re gonna be thinking about it. Now sometimes I’m like, “Oh, yeah,” like, if I eat a plate of vegetables, I’m like, “Mm.” I can feel—like, it’s not as rewarding as cake, right? Like …
Feltman: Sure, yeah.
Kaelberer: [Laughs] Let’s all be honest about that. But I’m like, “Mm.” I feel the satisfaction that my gut is like, “You made a good decision.”
Feltman: [Laughs] I love that. Thanks so much for coming on.
Kaelberer: Of course. Thank you so much.
Feltman: That’s all for today’s episode. We’ll be back on Friday to talk about how social media algorithms are shaping the future of language.
For Scientific American, this is Rachel Feltman. See you next time!
Rachel Feltman is former executive editor of Popular Science and forever host of the podcast The Weirdest Thing I Learned This Week. She previously founded the blog Speaking of Science for the Washington Post.
Fonda Mwangi is a multimedia editor at Scientific American and producer of Science Quickly. She previously worked at Axios, The Recount and WTOP News. She holds a master’s degree in journalism and public affairs from American University in Washington, D.C.
Alex Sugiura is a Peabody and Pulitzer Prize–winning composer, editor and podcast producer based in Brooklyn, N.Y. He has worked on projects for Bloomberg, Axios, Crooked Media and Spotify, among others.
If you enjoyed this article, I’d like to ask for your support. Scientific American has served as an advocate for science and industry for 180 years, and right now may be the most critical moment in that two-century history.
I’ve been a Scientific American subscriber since I was 12 years old, and it helped shape the way I look at the world. SciAm always educates and delights me, and inspires a sense of awe for our vast, beautiful universe. I hope it does that for you, too.
If you , you help ensure that our coverage is centered on meaningful research and discovery; that we have the resources to report on the decisions that threaten labs across the U.S.; and that we support both budding and working scientists at a time when the value of science itself too often goes unrecognized.
In return, you get essential news, captivating podcasts, brilliant infographics, , must-watch videos, challenging games, and the science world’s best writing and reporting. You can even gift someone a subscription.
There has never been a more important time for us to stand up and show why science matters. I hope you’ll support us in that mission.
Thank you,
David M. Ewalt, Editor in Chief, Scientific American
Source: www.scientificamerican.com